Have you watched the short video clip linked above? This is microbially generated gasoline ("mFuel") with just atmospheric distillation left to be done for it to be fit for gasoline (without the additives).
Leaving all matters upstream aside and looking only at the refinery processes that generate our transportation fuels, fuel refinery internal energy consumption has increased two to three times since the 1980s because of mandated product quality improvements and demand changes, with little potential to offset these through efficiency improvements. Of course, this additional energy use goes hand in hand with the corresponding CO2 emissions, as most energy used in refineries is thermal. Those developments became clear already in 1995, see https://www.concawe.eu/wp-content/uploads/2017/01/rpt695ocr-2006-00247-01-e.pdf.
It is important to get an understanding how big the absolute figures we are looking at here really are. E.g., the U.S. refineries in 2018 used 3,728TBTU of energy. Considering that the 'average' residential 2021 electricity customer in the U.S. used 10,632kWh p.a., refineries use and lose around the same amount of energy in electricity terms as 10.3 million residential customers draw p.a.!
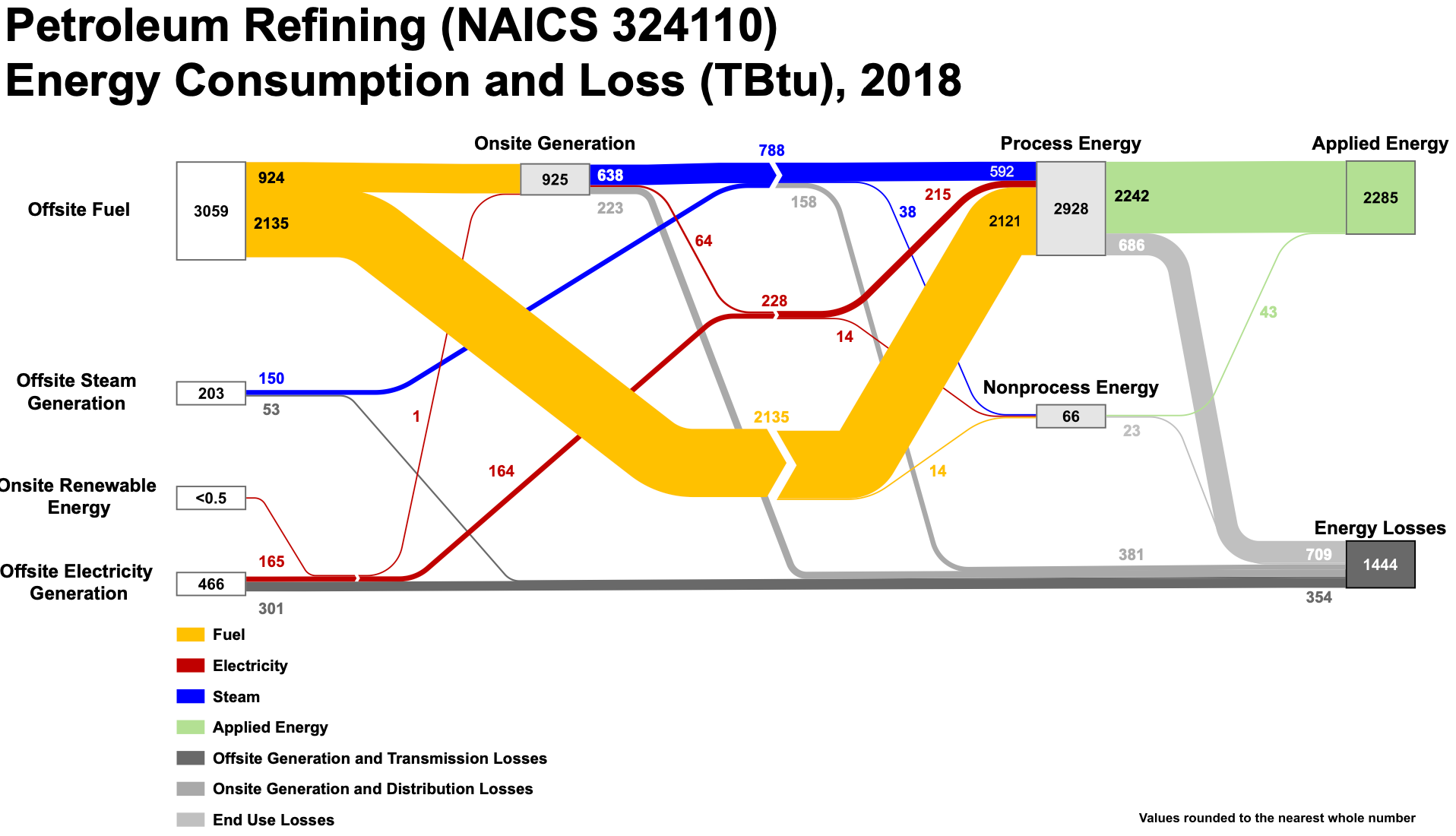
Those really significant numbers raise one question: Is there a way to produce at least some of the key refinery products, say transportation grade gasoline (U.S. 2022: 49% of 100% output) and diesel (U.S. 2022: 26% of 100% output) with less energy use and CO2 emissions?
And the answer is... yes! This can be done on large scale with the microbial technology discussed here–see my previous (and yet to come) posts for more details. So, in my definition, 'mFuels' means light ends generated solely by microorganisms from heavy hydrocarbon molecules of any origin, such as, e.g., crude oil, hydrocarbon wastes and petroleum sludge.
Our technology has been proven to work with actual feedstock samples from around the world–e.g., crudes from Saudi Arabia, the U.S., and Canada; synthetic engine and gearbox oils, axle grease, bunker fuels, ... We are now working on a scaled down 1000l/d pilot plant that uses the same process flow as envisaged for commercial size layouts.

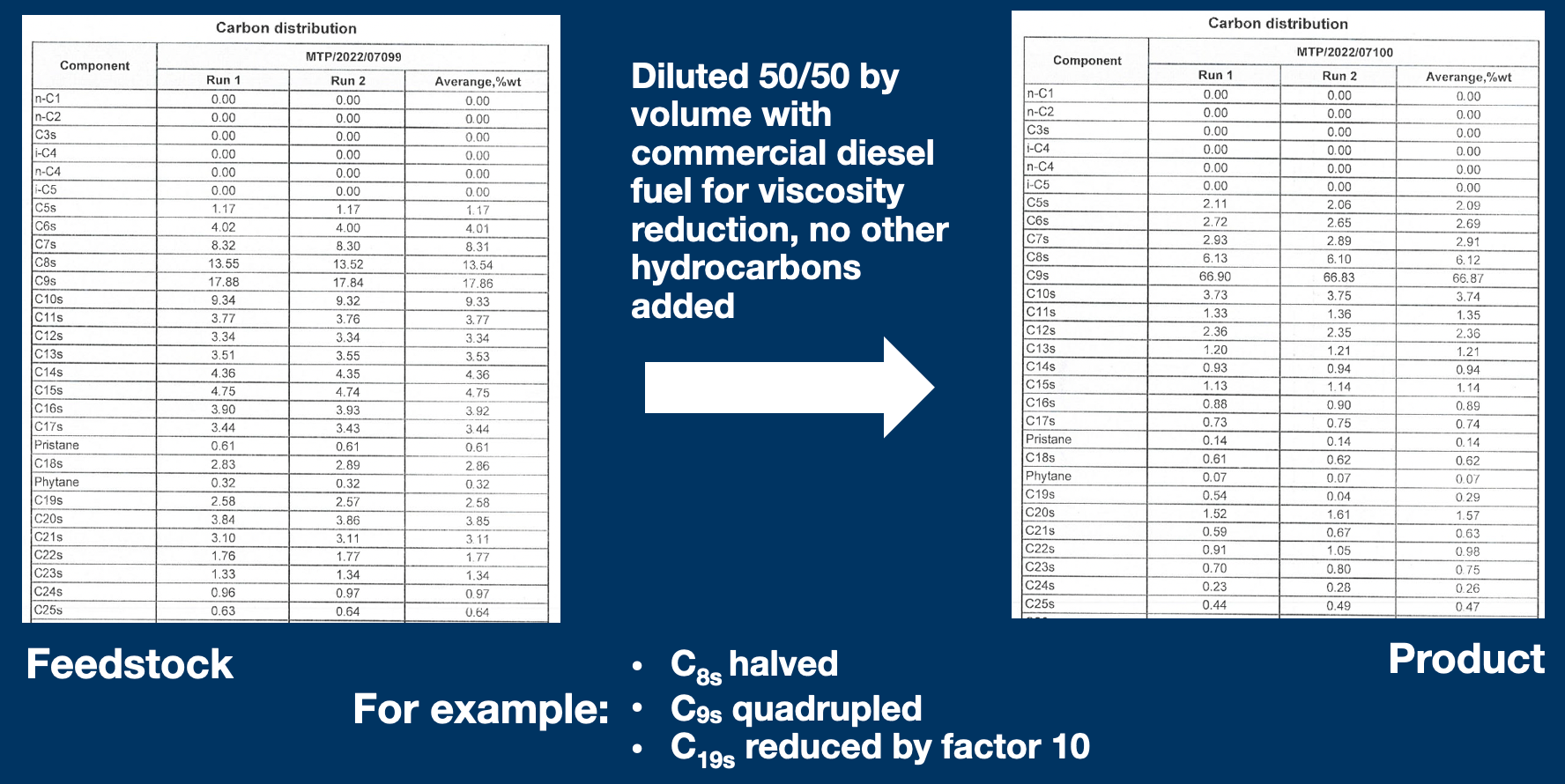
NB: The product can be further refined simply by allowing for more reaction time, liquid extraction ('washing') or atmospheric distillation. More details in the next post(s).
This would be a perfect transitional technology and could be upscaled starting with waste hydrocarbons—supported by respective legislation worldwide, see e.g. Europe https://environment.ec.europa.eu/topics/waste-and-recycling/waste-oil_en and Australia https://www.dcceew.gov.au/environment/protection/used-oil-recycling/product-stewardship-oil-program.
mFuels production doesn't need 10% to 15% of its feedstocks' energy content to run the process like conventional refineries do—it is a low temperature and low pressure process with very little CO2 emissions and will do with a fraction of it. It also leaves no non-distillable heavy hydrocarbon byproducts or tails, such as pitch, coke and distillation residues. If the conditions are right and substrates ('food') are plentiful, microbes will enter into an exponential growth phase that keeps going until conditions become unfavorable or substrate runs out. That makes the technology really fast—results like the above are reached within hours, not days.
With our technology, little CO2 is generated during manufacturing of transportation fuels. This is great progress in refinery operations, no matter if looking at waste hydrocarbon refineries or crude oil refineries.
I hope this made some interesting reading for you; more to come!

